Obesity, defined as abnormal or excessive fat accumulation (adiposity) presents a risk for excess morbidity as well as mortality. Obesity is traditionally measured through a simpler and reliable ratio termed as body mass index (BMI) which is expressed as weight in kilograms divided by square height in meters (kg/m2).
BMI has been found to strongly correlate with the gold standard methods for measuring body fat. Over several decades, BMI has proved itself as simpler, consistent, and reliable method for clinicians, healthcare professionals, insurance companies, and researchers to screen individuals who are at a greater risk of health problems due to their weight.
BMI is considered as one of the important vital signs in all medical records and all forms of insurance and life settlement applications screened for underwriting. Individuals are normally considered to be obese if they are 20% over the average weight or have a BMI of >30 kg/m2. [1]
Note: We use obesity and high BMI (which is defined as BMI of >30 kg/m2) concurrently in this review article below. For simplicity of reading, we use the BMI numbers without unit in this article.
Based on the BMI, obesity is further classified as:
- 30 to 34.9: Class 1/Grade I
- 35 to 39.9: Class 2/Grade II
- >40: Class 3/Grade III
A release from global burden disease (GBD) study [2] published in 2017 noted that the diseases related to high BMI caused 2.4 million deaths and 9.7 million disability-adjusted life years (DALYs = sum of the years of life lost due to premature mortality and the years lived with a disability due to the prevalent disease or condition) worldwide in 2017. The GBD study also observed a higher death rate and DALY rate among individuals in the age group of 45 to 90 years with high BMI. See Fig 1.
Fig. 1. Age-specific numbers and rates of deaths and DALYs attributable to high body mass index by sex, in 2017 (A) Deaths (B) DALYs – Disability-adjusted life year [2]

Large observational studies have noted that obesity predisposes individuals to many clinical conditions including, type 2 diabetes, cardiovascular diseases (heart attack, heart failure), chronic kidney disease, site-specific cancers, musculoskeletal disorders, mental and behavioral disorders, and infections [3] [4]. Studies also suggest that obesity leads to disease clustering, frailty, and poor health related quality of life [5] [6]. A recent observational study published in The Lancet [7] that analyzed UK biobank data and two Finnish cohorts revealed that high BMI is associated with:
- 4- to 12-fold risk of diabetes,
- 4- to 6-fold risk of sleep disorders,
- 3- to 4-fold risk of heart failure,
- 4- to 5-fold risk of gout, and
- >2-fold risk of hypertension, pulmonary embolism, deep vein thrombosis, renal failure, osteoarthritis, kidney cancer and bacterial infections.
The authors of this study also observed a linear relationship of obesity with multimorbid diseases (two obesity-associated conditions) and complex multimorbid diseases (more than two multi-morbid conditions). The risk of developing four complex comorbid conditions was found to be 4-fold in individuals classified with class 1 obesity (BMI 30 to 34.9), 6-fold in class 2 obesity (BMI 35 to 39.9), and 10-fold in class 3 obesity (BMI >40). The risk of developing these complex multimorbid diseases starts early at the age of 45 to 55 years in obese individuals compared to age of 55 to 65 years in healthy weight (BMI 20 to 30) individuals.
Obesity and Mortality
The relationship of BMI and mortality can be best explained with a ‘J-shaped’ curve which indicates that the excess mortality is being observed in underweight (BMI <20), overweight (BMI 25 to 30), and obese individuals (BMI >30). However, in elderly individuals the excess mortality has been observed in underweight individuals (BMI <20) and individuals with obesity class/grade 2 and 3 (BMI >35).
Insured Lives/Insurance population
Generally, applicants for life insurance tend to be younger (age 18-50 years) and healthier which could result in a better survival experience compared to the survival that is observed in the general population. Additionally, screening of healthy applicants and risk classification through the process of underwriting may result in improved survival experience for insurance companies, also referred to as underwriting selection effect. All such improved survival experiences that distinguish insurance applicants from the general population are then incorporated in the pricing of life insurance policies. As discussed earlier, BMI is one of the risk factors that is commonly used by insurance companies for underwriting of its applicants.
A study published in the Journal of Insurance Medicine [8] of 356,000 life insurance applicants from the insurance and reinsurance companies of Lincoln Financial Group observed that BMI of 31 to 33 was associated with a 1.75-fold risk of excess mortality and a BMI of >34 was associated with a 1.8-fold risk of excess morality in comparison to insurance applicants with a BMI of 20 to 25: Thus indicating that a higher BMI poses an increased risk of mortality.
General Population
Several studies amongst the general population have evaluated the burden of excess mortality and life expectancy in the general population and have concluded that a higher BMI leads to excess mortality in comparison to BMI of 20 to 25. However, the results from these studies vary based on the socio–demographic factors that include geography, age, sex, smoking, and presence of comorbid conditions.
Global BMI mortality collaboration study [8] that has reviewed the relationship of BMI and mortality in more than 10 million individuals noted that excess mortality due to high BMI is slightly lower in the North American general population in comparison to other continents, see Fig. 2.
Fig. 2. Association of BMI with all-cause mortality by geographical region – never smokers without pre-existing chronic disease (n = 10,625,411) [8]
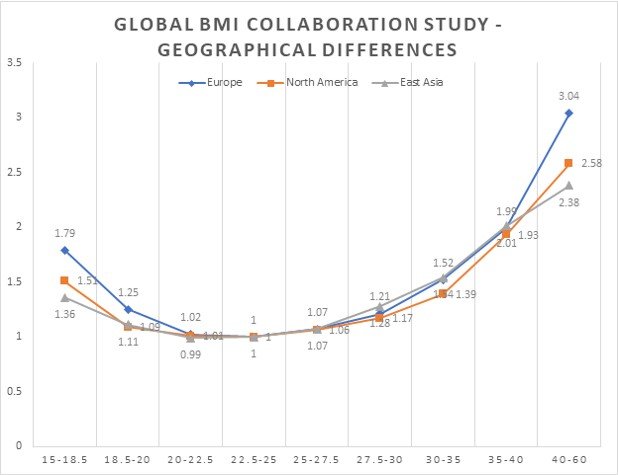
It noted that with every 5 units of BMI of >25 the excess mortality increased by 1.5-fold in men and 1.3-fold in women [9]. The authors of this study also observed a ‘J’ shaped relationship between BMI and all-cause mortality, i.e., excess mortality was noted in both underweight (BMI <22.5) as well as overweight (BMI >25.0) individuals.
Similar findings have been noted in a study [10] that analyzed 1.46 million white men and women from the US. It observed that the hazard ratio for all-cause mortality in healthy white men who never smoked ranged from 1.03- to 3-fold in men (BMI 27.5 to 45) and 1.03- to 2.5-fold in healthy women who never smoked (BMI of 25 to 45). See detailed estimates in Fig. 3.
Fig. 3. Estimated hazard ratios for death from any cause according to BMI for all study participants (1.46 million white men and women of age 19-84 years) and for healthy subjects who never smoked [10]

Risk Factors Associated with BMI and Mortality
Mortality in obese individuals is multifactorial and is dependent on variables that include geography, age, sex, smoking, and presence of cardiometabolic risk factors (e.g.: hypertension, diabetes, coronary artery disease, and stroke).
These factors act as continuous variables and contribute to varying mortality experiences. Studies discussed below explore this relationship and estimate the impact of these interdependent factors.
Age and BMI
Young obese individuals in the age group of 20 to 49 years have been found to have a higher risk of mortality in the range 1.7- to 4-fold compared with individuals in the age group of 60 to 84 years, range 1.2- to 2-fold. [10]
Table 1. Estimated hazard ratios for death from any cause among healthy subjects who never smoked (1.46 million white men and women) according to BMI and age at baseline
Age Group (In years) | BMI | ||
| 30.0-34.9 | 35.0-39.9 | 40.0-49.9 | |
| 20-49 | 1.79 (1.61-1.99) | 2.48 (2.14-2.88) | 3.70 (3.03-4.50) |
| 50-59 | 1.56 (1.46-1.68) | 2.06 (1.86-2.28) | 2.77 (2.42-3.16) |
| 60-69 | 1.34 (1.28-1.41) | 1.77 (1.64-1.91) | 2.27 (2.03-2.53) |
| 70-84 | 1.24 (1.12-1.38) | 1.59 (1.33-1.90) | 1.91 (1.44-2.52) |
Sex and BMI
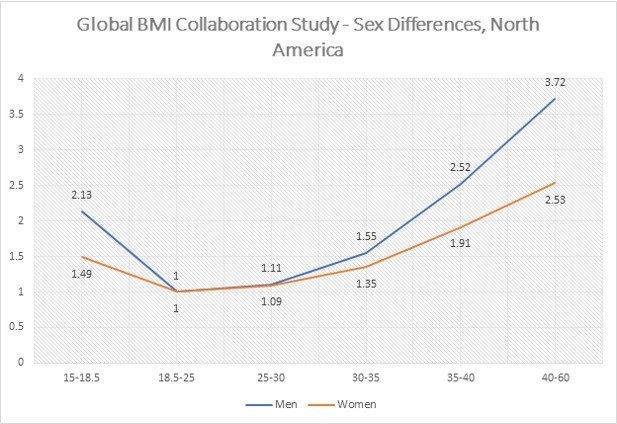
Several studies have observed the relationship of sex with BMI for all-cause mortality. A meta-analysis of 293 studies with more than 10 million participants [9] observed that females tend to have a slightly lower all-cause mortality (approximately 10 to 15%) in comparison to males, see Fig. 4. Similar findings have been observed in a study of individuals from the US who were of age >70 years [11].
Fig. 4. Association of BMI with all-cause mortality by sex in North America [9]
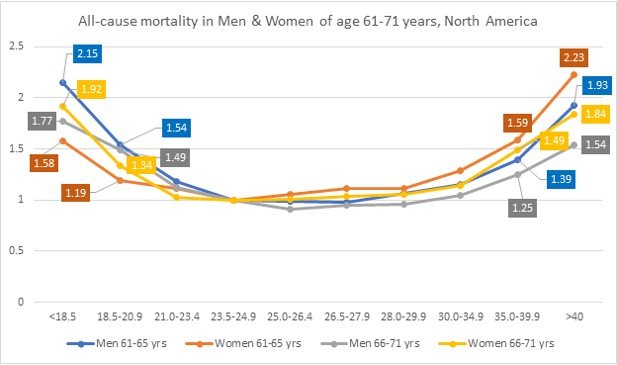
However, a study comprising of US men and women of age 50 to 71 years [12] observed that that males have a slightly lower all-cause mortality compared to females, thus indicating age and sex plays an interdependent role with BMI. See Fig. 5.
Fig 5. Relative risk of death in men and women (527,000 US men and women) according to age after adjusting for race or ethnic group, level of education, alcohol consumption and physical activity [12]
A study of 12.8 million Korean adults in the age group of 18 to 99 years which focused on sex and age-specific association of BMI with all-cause mortality [13] observed that females with high BMI >30 in the age group of 45-74 years had lower or similar excess all-cause mortality compared to males with BMI of >30. However, the authors observed that females in the age group of 18 to 44 years and 75 to 99 years with BMI of >30 had a higher all-cause mortality compared to males for BMI of >30. The findings from the Korean study signify the impact of geography, age, sex, and genetics with BMI on all-cause mortality.
Smoking and BMI
Association of smoking with BMI on all-cause mortality is complex and mired with controversy based on the underlying statistical models used by studies to estimate the relationship with mortality. The authors Banack et al, explain this relationship in a prospective cohort study [14] from four communities in the US where they examined the relationship of smoking and obesity with mortality. They noted that the studies [12] [13] that have probably used the multiplicative interaction model, (the product of the joint effects of obesity and smoking is greater (or less) than the product of two exposures individually) noted that obese current or ever smokers have a lower relative risk of death compared with obese never smokers (see Fig. 6).
Fig. 6. Study with multiplicative model that shows obese smokers have a lower relative risk of death compared with obese non-smokers [12] 

However, studies [15] [16] [17] that have probably used the additive interaction model, (the number of deaths caused by the combination of obesity and smoking is greater (or less) than the sum number of deaths that would be caused independently by either exposure) have noted that obese current or ever smokers have a higher relative risk of death compared with obese never smokers (see Fig. 7). Banack et al, in their analysis utilized the additive interaction model and observed that the incidence rate ratio (IRR) of all-cause mortality for smoking among non-obese participants was 2.00 (95% CI 1.79-2.24), IRR for obesity among non-smokers was 1.31 (95% CI, 1.31-1.51), and the IRR for the joint effect of smoking and obesity on mortality was 1.97 (95% CI, 1.73-2.22). The findings from these studies suggest that smoking is an independent risk factor that has a higher risk of all-cause mortality compared to obesity alone (2.00 vs. 1.31) and jointly this has an effect which is greater than obesity alone (1.97 vs 1.31). Studies have also observed that the risk of all-cause mortality in underweight (BMI <18) smokers is significantly increased, 1.2- to 3-fold compared to underweight never smokers.
Fig. 7. Study [16] that shows obese current smokers have a higher relative risk of death compared with obese non-smokers


Insurance applicants that smoke or use tobacco are commonly charged 50 to 80% higher base premium compared with non-smokers for the independent risk conferred by smoking on mortality for several reasons.
To summarize on the risk factors of mortality with BMI – younger (age <65 years) obese individuals (age <65 years) have higher excess mortality compared to elderly obese individuals (age >65 years); obese females have lower mortality compared to obese males; and smokers (both underweight and obese) have higher mortality compared to non-smokers.
Drivers of Mortality in Obese Individuals
Obesity is known to be an underlying cause and/or one of the risk factors in development of cardiometabolic illnesses (hypertension, diabetes, atherosclerosis), digestive, respiratory, neurological, musculoskeletal, infectious diseases, and death. An observational study [7] that used prospective data from two Finnish cohorts and the UK biobank with >600,000 participants observed that the risk of developing these illnesses with obesity (BMI >30) ranged from two-fold to 12-fold. (See Table 2.)
Table 2. Associations between obesity and incidence of diseases [7]
| Disease category (as per ICD) | Hazard Ratio (95%I) in Finnish cohorts | Hazard Ratio (95%I) in UK Biobank cohort |
| Diabetes | 12.14 (11.24-13.11) | 4.53 (3.93-5.23) |
| Sleep disorders | 6.27 (5.68-6.92) | 4.10 (3.63-4.62) |
| Heart failure | 4.17 (3.35-5.20) | 3.24 (2.96-3.55) |
| Hypertension | 3.20 (3.00-3.41) | 1.98 (1.77-2.21) |
| Renal failure | 2.95 (2.27-3.83) | 2.32 (2.13-2.52) |
| Pulmonary embolism | 2.86 (2.27-3.60) | 2.34 (2.15-2.54) |
| Deep vein thrombosis | 2.43 (1.96-3.03) | 2.07 (1.89-2.26) |
| Bacterial infections | 2.16 (1.97-2.37 | 1.43 (1.37-1.48) |
| Asthma | 1.95 (1.79-2.12) | 2.23 (1.98-2.51) |
While there is a risk of developing chronic illnesses due to obesity; mortality in obese individuals happens to be driven by coronary heart disease, stroke, respiratory disorders, and cancer, as well as to a plausible extent by digestive, neurological, musculoskeletal, and infectious diseases. The Global BMI Mortality Collaboration (a large meta-analysis of 239 prospective studies) [9] observed that the risk of death due to BMI is primarily driven by coronary heart disease, stroke, respiratory, and cancer which starts to increase with BMI of >27. Obese individuals are at a higher risk of death; 2- to 4-fold due to coronary heart disease, 1.5- to 3-fold due to stroke, 1.5- to 3.5-fold due to respiratory disorders, and 1.25- to 2-fold due to cancer. See Fig. 8.
Fig 8. Association of BMI with cause-specific mortality [9]

Obesity results in an increased incidence ranging 2-fold to 10-fold of diabetes, sleep disorders, heart failure, hypertension, renal failure, pulmonary embolism, deep vein thrombosis, bacterial infections, and asthma. Mortality due to obesity is primarily driven by cardiometabolic disorders with most deaths occurring due to diabetes and its complications, coronary artery disease/heart attack, and stroke.
Obesity Paradox in Geriatric Population
Obesity and its impact on mortality in geriatric population remains a topic of interest for clinicians, researchers, and insurance medicine professionals. Obesity has been found to be independently associated with greater limitations in activities of daily living and larger increase in functional impairments [18]. Studies have found that disability-free life expectancy in elderly individuals was greatest in individuals with BMI of 25-30 and those with BMI of >30 were significantly more likely to experience disability. [19] Obesity has also been found to be linked with increased hospitalizations and surgeries [20]. However, the impact of obesity on mortality and weight loss interventions in elderly individuals remains a controversial topic. Studies have shown that increased adiposity may have a protective effect in older adults, often referred to ‘the obesity paradox’. Studies have observed that being overweight (BMI 30 to 35) could be associated with lower mortality in older adults compared to all other BMI ranges (BMI <30 and >35) [21] [11]. Findings from these studies are supportive of the hypothesis that increased adiposity helps during frailty periods. They also support the correlative rather than causal effect relationship that though the presence of obesity increases the risk of developing chronic conditions such as end-stage renal disease and chronic heart failure, it does not predispose them to the development of advanced cancers. However, once these chronic cardiometabolic conditions are present, the obese elderly adults tend to have higher survival rates; mostly attributed to the presence of larger stores of body mass (therefore energy) as well as a better overall nutritional status.
A population-based study [22] that followed up Mexican American men and women aged 75 and older in the U.S. for 12-years observed that individuals with BMI of 25 to 35 (overweight + class 1/grade I obesity) had a lower mortality compared to individuals with an optimal BMI of 20 to 25. However, individuals with morbid obesity, i.e., class 2/grade II obesity (BMI of >35) and underweight (BMI <20) were found to have a higher mortality compared to individuals with BMI of 20 to 35. See Fig. 9. Another study of Caucasian seniors from Poland observed BMI of 35 to 39 in women and BMI of 30 to 35 in men was associated with lowest all-cause mortality [23].
Fig 9. Relationship of BMI with survival in 1,416 Mexican American men and women aged >75 years [23]

A study [24] of 16,837 elderly individuals that were hospitalized for stroke in the U.S. observed that individuals with BMI of 26 to 39 had a lower risk of in-hospital mortality compared to BMI of <25 and >40. See Fig. 10.
Fig 10. The relationship between BMI and in-hospital mortality [24]

An overview of obesity paradox in cardiovascular diseases [25] observed that elderly individuals with class 1/grade I BMI (30 to 35) had lowest cardiovascular disease (CVD) specific mortality compared to all other BMI ranges. It noted that elderly individuals diagnosed with coronary artery disease (CAD) and BMI of 25 to 35 had a favorable prognosis and demonstrated lower risk of CVD and all-cause mortality compared with underweight and optimal BMI (20 to 25) individuals who were also diagnosed with CAD. However, these observations were limited to elderly individuals with BMI of 25 to 35. Individuals with BMI of >35 were found to have significantly elevated CVD-specific as well as all-cause mortality.
Obesity paradox to some extent is supported by hypothesis and studies; however, these relationships are complex and remain unadjusted for several confounders and comorbid impairments noted in elderly individuals.
Outlook
Newer studies [26] have proposed definitions of metabolically healthy overweight (MHO) individuals based on multifactorial criteria of cardiometabolic risk factors. These studies differentiate individuals as metabolically healthy based on limits/cut-offs for systolic blood pressure, lipids, waist to hip ratio, and prevalent diabetes and hypertension. MHOs have been found to have better survival rates compared to metabolically unhealthy overweight (MUO) individuals. In future, we may see more research studies that explore these multifactorial relationships that impact mortality in obesity.
Dr. Rahul Nawander is a Medical Director at Fasano Associates
Any views expressed in this article are those of the author(s) and do not necessarily reflect the views of Life Risk News or its publisher, the European Life Settlement Association.
Footnotes:
[1]Obesity Basics, Centers for Disease Control and Prevention. Available at https://www.cdc.gov/obesity/basics/index.html
[2]Dai, Haijiang, et al. “The global burden of disease attributable to high body mass index in 195 countries and territories, 1990–2017: An analysis of the Global Burden of Disease Study.” PLoS medicine 17.7 (2020): e1003198. Available at https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7386577/
[3]Blüher, Matthias. “Obesity: global epidemiology and pathogenesis.” Nature Reviews Endocrinology 15.5 (2019): 288-298. Available at https://pubmed.ncbi.nlm.nih.gov/30814686/
[4]Heymsfield, Steven B., and Thomas A. Wadden. “Mechanisms, pathophysiology, and management of obesity.” New England Journal of Medicine 376.3 (2017): 254-266. Available at https://pubmed.ncbi.nlm.nih.gov/28402780/
[5]Strandberg, T. E., et al. “Association of midlife obesity and cardiovascular risk with old age frailty: a 26-year follow-up of initially healthy men.” International journal of obesity 36.9 (2012): 1153-1157. Available at https://pubmed.ncbi.nlm.nih.gov/22614054/
[6]Agborsangaya, Calypse B., et al. “Multimorbidity prevalence in the general population: the role of obesity in chronic disease clustering.” BMC Public Health 13.1 (2013): 1-6. Available at https://pubmed.ncbi.nlm.nih.gov/24325303/
[7]Kivimäki, Mika, et al. “Body-mass index and risk of obesity-related complex multimorbidity: an observational multicohort study.” The lancet Diabetes & endocrinology 10.4 (2022): 253-263. Available at https://pubmed.ncbi.nlm.nih.gov/35248171/
[8]Niverthi, Murali, and B. Ianovic. “Body mass index and mortality in an insured population.” JOURNAL OF INSURANCE MEDICINE-NEW YORK- 33.4 (2001): 321-328. Available at https://pubmed.ncbi.nlm.nih.gov/11877912/
[9]Di Angelantonio, Emanuele, et al. “Body-mass index and all-cause mortality: individual-participant-data meta-analysis of 239 prospective studies in four continents.” The Lancet 388.10046 (2016): 776-786. Available on https://pubmed.ncbi.nlm.nih.gov/27423262/
[10]Berrington de Gonzalez, Amy, et al. “Body-mass index and mortality among 1.46 million white adults.” New England Journal of Medicine 363.23 (2010): 2211-2219. Available at https://pubmed.ncbi.nlm.nih.gov/PMC3066051
[11]Allison, David B., et al. “Body mass index and all-cause mortality among people age 70 and over: the Longitudinal Study of Aging.” International journal of obesity 21.6 (1997): 424-431. Available at https://pubmed.ncbi.nlm.nih.gov/9192224/
[12]Adams, Kenneth F., et al. “Overweight, obesity, and mortality in a large prospective cohort of persons 50 to 71 years old.” New England Journal of Medicine 355.8 (2006): 763-778. Available at https://pubmed.ncbi.nlm.nih.gov/16926275/
[13]Yi, Sang-Wook, et al. “Sex-age-specific association of body mass index with all-cause mortality among 12.8 million Korean adults: a prospective cohort study.” International Journal of Epidemiology 44.5 (2015): 1696-1705. Available at https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4681110/
[14]Banack, Hailey R., and Jay S. Kaufman. “Estimating the time-varying joint effects of obesity and smoking on all-cause mortality using marginal structural models.” American journal of epidemiology 183.2 (2016): 122-129. Available at https://pubmed.ncbi.nlm.nih.gov/26656480/
[15]Meyer, Haakon E., et al. “Body mass index and mortality: the influence of physical activity and smoking.” Medicine & science in sports & exercise 34.7 (2002): 1065-1070. Available at https://pubmed.ncbi.nlm.nih.gov/12131242/
[16]Freedman, D. Michal, et al. “The mortality risk of smoking and obesity combined.” American journal of preventive medicine 31.5 (2006): 355-362. Available at https://pubmed.ncbi.nlm.nih.gov/17046405/
[17]Aune, Dagfinn, et al. “BMI and all cause mortality: systematic review and non-linear dose-response meta-analysis of 230 cohort studies with 3.74 million deaths among 30.3 million participants.” bmj 353 (2016). Available at https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4856854/
[18]Chen, Honglei, and Xuguang Guo. “Obesity and functional disability in elderly Americans.” Journal of the American Geriatrics Society 56.4 (2008): 689-694. Available at https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2391089/
[19]Al Snih, Soham, et al. “The effect of obesity on disability vs mortality in older Americans.” Archives of internal medicine 167.8 (2007): 774-780. Available at https://pubmed.ncbi.nlm.nih.gov/17452539/
[20]Etzioni, David A., et al. “The aging population and its impact on the surgery workforce.” Annals of surgery 238.2 (2003): 170. Available at https://www.ncbi.nlm.nih.gov/pmc/articles/PMC1422682/
[21]Flegal, Katherine M., et al. “Association of all-cause mortality with overweight and obesity using standard body mass index categories: a systematic review and meta-analysis.” Jama 309.1 (2013): 71-82. Available at https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4855514/
[22]Jadhav, Reshma, Kyriakos S. Markides, and Soham Al Snih. “Body Mass Index and 12-year Mortality Among Older Mexican Americans Aged 75 Years and Older.” Innovation in Aging 4.Suppl 1 (2020): 919. Available at https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7741648/
[23]Puzianowska-Kuznicka, Monika, et al. “Obesity Paradox in Caucasian Seniors: Results of the PolSenior Study.” The Journal of Nutrition, Health & Aging 23.9 (2019): 796. Available at https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6800404/
[24]Rozen, Guy, et al. “The obesity paradox in real-world nation-wide cohort of patients admitted for a stroke in the US.” Journal of Clinical Medicine 11.6 (2022): 1678. Available at https://pubmed.ncbi.nlm.nih.gov/35330003/
[25]Elagizi, Andrew, et al. “An overview and update on obesity and the obesity paradox in cardiovascular diseases.” Progress in cardiovascular diseases 61.2 (2018): 142-150. Available at https://pubmed.ncbi.nlm.nih.gov/29981771/
[26]Zembic, Anika, et al. “An empirically derived definition of metabolically healthy obesity based on risk of cardiovascular and total mortality.” JAMA Network Open 4.5 (2021): e218505-e218505. Available at https://pubmed.ncbi.nlm.nih.gov/33961036/
Additional Reading:
- Lin, Wen-Yuan, et al. “Body mass index and all-cause mortality in a large Chinese cohort.” Cmaj 183.6 (2011): E329-E336. Available at https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3071414/
- Lin, Gen-Min, et al. “The obesity-mortality paradox in elderly patients with angiographic coronary artery disease: a report from the ET-CHD registry.” Acta Cardiologica 70.4 (2015): 479-486. Available at https://pubmed.ncbi.nlm.nih.gov/26455252/
- Sun, Yi-Qian, et al. “Body mass index and all cause mortality in HUNT and UK Biobank studies: linear and non-linear mendelian randomisation analyses.” bmj 364 (2019). Available at https://pubmed.ncbi.nlm.nih.gov/30957776/
- Malenfant, Jason H., and John A. Batsis. “Obesity in the geriatric population–a global health perspective.” Journal of global health reports 3 (2019). Available at https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8136402/
- Liu, Zuolu, et al. “Adiposity and outcome after ischemic stroke: obesity paradox for mortality and obesity parabola for favorable functional outcomes.” Stroke 52.1 (2021): 144-151. Available at https://pubmed.ncbi.nlm.nih.gov/33272129/
- Loos, Ruth JF, and Giles SH Yeo. “The genetics of obesity: from discovery to biology.” Nature Reviews Genetics 23.2 (2022): 120-133. Available at https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8459824/

